The ratio of ΔH cal to ΔH VH could be used for study of intermediate folding states which occur near the melting point. Founded on a robust collaborative scientific process and rigorously tested in laboratories around the globe USP Reference Standards are trusted as.
Differential Scanning Calorimetry Covalent Metrology Analytical Labs

Polymers Polyethylene Terephthalate Pet Netzsch Analyzing Testing

Melting Point Determination On Palladium Netzsch Analyzing Testing
There are several important parameters in performing DSC on nucleic acids DNA or RNA.
Dsc melting point. The International Union of Pure and Applied Chemistry IUPAC ˈ aɪ juː p æ k ˈ juː- is an international federation of National Adhering Organizations that represents chemists in individual countries. Whether youre performing QAQC applications studying processes in polymers or pharmaceuticals or developing the cures of tomorrow our new DSC platforms will open your eyes to a world of exciting new opportunities. Discover studio one two and three-bedroom apartments in Herndon VA that put you close to everywhere you want to be.
Impure and poly-meric samples whose melting curves are. The extent of crystallinity of the NLCs and presence of liquid phase inside the matrix of solid lipid can also be confirmed through DSC study. Differential scanning calorimetry DSC.
Simple melting point apparatus. Impure or polymeric samples will display more concave sides and longer tails while. Crystallization and melt transitions.
Using a DSC for this task gives you the melting temperature from a calibrated and highly precise system. Materials that decompose before the melting point. DSC performed with the microscope accessory has several applications that may prove insightful to better understanding materials properties and behaviors.
However the number is often imprecise and difficult to reproduce. Curie point measurements of Ferromagnetic Materials DSC Heat Flow Cell Constant Calibration. Although polyolefin is regarded as material with high crystallinity and thus difficult adhesive material we recommend the use of HARDLENAdhesion Promoter to Polyolefins to improve the adhesive property for spray and gravure coating.
This makes it difficult to pinpoint the exact location of the peak maximum as well as the start and end points which define the melting point and the melting range respectively. When you measure the melting point Tm in a DSC you get not only the onset. Melting endotherms of high purity metal standards like Zinc Indium Gold.
Explore and purchase our latest Reference Standards and discover whats coming. Calibrating the heat flow response of a DSC by recording the melting endotherm of a high-purity standard material as a function of time. DSC can be used to study the melting of a crystalline polymer or the glass transition.
This website uses cookies to help provide you with the best possible online experience. DSC performed with the microscope accessory has several applications that may prove insightful to better understanding materials properties and behaviors. The melting points of pure materials are often characterized by an almost straight or linear line on the low temperature side of the peak.
The amount of magnesium stearate was varied and the following methods were used for microsphere evaluation. Also some chemical reactions in solid phase result in gaseous weight loss ex. For each cycle a new sample of ILZIF-8 was produced by placing the starting batch into the same Pt crucible as used for the subsequent DSC experiment and melting using the HT condition with a.
Differential scanning calorimetry DSC is a thermoanalytical technique in which the difference in the amount of heat required to increase the temperature of a sample and reference is measured as a function of temperature. Plots showing differences in heat flow between a sample and reference as a function of time or temperature yield information on thermal transitions in a sample due to melting. It is a member of the International Science Council ISC.
It also gains you considerably more information about the sample. The DSC set-up is composed of a measurement chamber and a computer. More about Thermal Analysis.
42 44 Therefore the DSC calorimeter is ideal for determining ΔC p measuring C p directly. IUPAC is registered in Zürich Switzerland and the administrative office known as the IUPAC Secretariat is in Research. The innovative product range includes Differential Scanning Calorimeters DSC Thermogravimetric Analyzers TGA Thermomechanical Analyzer TMA and Dynamic Mechanical Analyzers DMA as well as an unmatched very powerful common Thermal Analysis software platform.
Mass changes as function of pressure. Heat difference Pressurized TGA PTGA. The conventional DSC plot does not have a well-defined peak shape.
Whether you are working playing or taking on new adventures youre at the perfect starting point. Interpreting DSC Data 8 Melting Point Peaks Melting peak characteristics will vary in response to many variables. Crystallization and melt transitions.
Na 2CO 3 s SiO. Please read our Terms Conditions and Privacy Policy for information about. It allows the scientist to capture images of.
Melting crystallization and mesophase transitions The heat of fusion and the melting point can be determined from the melting curve. Melting Point Dropping Point Determination. Powder melting point DSC 202 C 396 F ASTM D3418 Particle size 54 μm ASTM D3451 Bulk density of powder 048 gcm3 0017 lbin3 ASTM D1895 HP 3D High Reusability PA 11 ideal for producing ductile8 quality parts.
H 2O CO CO 2 SO x NO x Cl 2 F 2 CH 3OH etc. Di erential scanning calorimetry DSC is a technique used to investigate the response of polymers to heating. 5 DSC Training Course-04-03-02-01 00 01 Heat Flow Wg 0 25 50 75 100 125 150 Exo Up Temperature C Endothermic Heat Flow Heat flows into the sample as a result of either.
Hydrochloride salt of FEX Form I has been reported to have a melting point of 193199 C 11. Both the sample and reference are maintained at nearly the same temperature throughout the experiment. Microspheres containing the mucoadhesive polymer chitosan hydrochloride with matrix polymer Eudragit RS pipemidic acid as a model drug and agglomeration preventing agent magnesium stearate were prepared by the solvent evaporation method.
Two pans are heated in the measurement chamber. Differential Scanning Calorimetry DSC is an analytical technique which measures the heat flow into or out of a sample as a function of time andor temperature. However the melting peak above the Tg shows dissimilarities between the two different techniques.
In cases of hot-melt processing. With pure substances where the low tem-perature side of the melting peak is almost a straight line Fig. See more than you ever thought possible with our advanced line of differential scanning calorimetry DSC solutions.
Enjoy the outdoors on one of the many local trails. Shop and dine with tons of options close by. Generally the temperature program for a DSC analysis.
The sample pan contains the. Differential scanning calorimetry DSC helps in an overview of the druglipid interactions status of the lipid and melting and recrystallization behaviors of the NLCs. 4a the melting point corresponds to the onset.
It allows the scientist to capture images. The melting point range of FEX free base Form II was found to be 225230 C by differential scanning calorimetry DSC. We provide materials that are suitable for painting plastics as well as print and adhesive processing.

Evaluating Physical Properties Of Polymer Materials

A Typical Dsc Thermogram To Determine The Freezing Point And Dh Of Ice Download Scientific Diagram

Differential Scanning Calorimetry
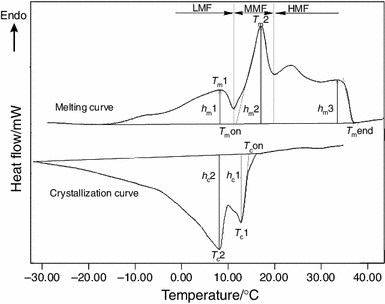
Melting And Crystallization Dsc Profiles Of Milk Fat Depending On Selected Factors Springerlink
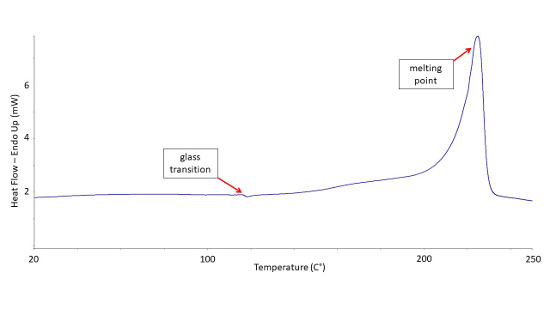
Differential Scanning Calorimetry Testing Dsc Analysis Microtrace
.jpg)
Quick Determination Of Polymer Crystallinity By Dsc
Differential Scanning Calorimetry Dsc Thermal Analysis Anderson Materials Evaluation Inc Anderson Materials Evaluation Inc
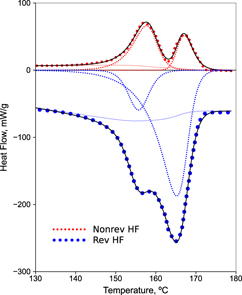
New Approach To The Double Melting Peak Of Poly L Lactic Acid Observed By Dsc Journal Of Materials Research Cambridge Core
