The way clinical trials are conducted in the EU will undergo a major transformation once the Clinical Trial Regulation comes into effect. Regulation and Clinical Trials.
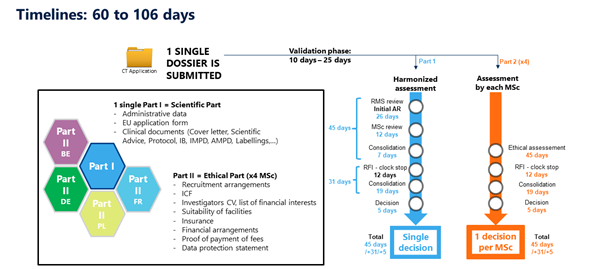
Eu Clinical Trials Regulation The Need For Coordination By Sponsors
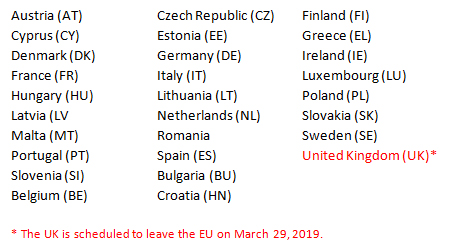
Initiating Multinational Clinical Trials Major Differences Between The Us And Eu

Figure 2 From Will The Eu Clinical Trials Regulation Support The Innovative Industry In Bringing New Medicines Faster To Patients Semantic Scholar
We regulate the use of therapeutic goods supplied in clinical trials in Australia under the therapeutic goods legislation.

Eu clinical trial regulation. The webinar covers the impending changes coming with the EU Parliament passage of the EU Clinical Trial Regulation which will affect all. Telephone 44 020 3660 6000. The EU Clinical Trials Register website has.
The EU Clinical Trials Register currently displays 41232 clinical trials with a EudraCT protocol of which 6757 are clinical trials conducted with subjects less than 18 years old. Clinical trial sponsors must be aware of the requirements to import export manufacture and supply therapeutic goods in Australia. An agency of the European Union.
This Regulation lays down rules concerning the placing on the market making available on the market or putting into service of medical devices for human use and accessories for such devices in the Union. Information on the authorisation conduct and. The Heads of Medicines Agencies HMA agreed in 2004 to establish a clinical trials facilitation group CTFG to coordinate the implementation of the EU clinical trials directive 200120 EC across the member statesIn relation to clincial trials the Clinical Trials Facilitation and Coordination Group CTFG acts as a forum for discussion to.
The EU Portal will be a single entry point for submission of data and information relating to clinical trials required by the Regulation. Clinical trials conducted in Australia are subject to various regulatory controls to ensure the safety of participants. The description of any phase II-IV adult clinical trial where the investigator sites are in the European Union or the European Economic Area.
30 Churchill Place Canary Wharf London E14 5EU United Kingdom. This Regulation also applies to clinical investigations concerning such medical devices and accessories conducted in the Union. Facsimile 44 020 3660 5555.
And other countries should keep an eye on how the regulation rolls out in early 2022 and its impact on data-sharing transparency and acceleration of trial startup even on trials conducted in the US. Transition between reporting to EU. As the EU transitions to its long-awaited new Clinical Trial Regulation CTR researchers site managers and trial coordinators in the US.
This makes it in particular difficult to perform a given clinical trial in several Member 2752014 EN Official Journal of. Articles 80 and 81 of the Regulation assigned the EMA the task of creating an EU Portal and Database. The Regulation will require.
Systems will enable sponsors and CROs to specify the type of report from a study and assign the study type as a clinical trial. The register also displays information on 18700 older paediatric trials in scope of Article 45 of the Paediatric Regulation EC No 19012006. And other countries should keep an eye on how the regulation rolls out in early 2022 and its impact on data-sharing transparency and acceleration of trial startup even on trials conducted in the US.
Guidance and regulation. Standard for the European Union EU Japan and the. However experience shows that a harmonised approach to the regulation of clinical trials has only been partly achieved.
Serious breaches sponsors clinical trials compliance clinical trial Regulation EU No 5362014 violations protocol regulation patients assessment. EU Clinical Trial Portal and Database. MDR - Medical Device Regulation - The medical device regulation MDR is a set of new requirements governing medical devices marketed in the European Union.
Consistent rules for conducting clinical trials throughout the EU. MDR aims to provide greater patient protection with more stringent requirements for clinical evidence greater transparency and closing loopholes for devices that were previously grandfathered in. The regulation will replace the existing Clinical Trials Directive 200120EC and will harmonise the registration assessment and supervision processes for clinical trials throughout the EU via the CTIS.
The EU Clinical Trials Register website puts these requirements into practice. The goal of the Clinical Trials Regulation is to create an environment that is favourable to conducting clinical trials in the EU with the highest standards of safety for participants and increased transparency of trial information. As the EU transitions to its long-awaited new Clinical Trial Regulation CTR researchers site managers and trial coordinators in the US.
The format and content of clinical trial protocols sponsored by pharmaceutical biotechnology or medical device companies in the United States European Union or Japan have been standardized to follow Good Clinical Practice guidance issued by the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use. Clinical Trial Environment Investigator Study Subjects US Food and Drug. What information can I find in the EU Clinical Trials Register.

Impacts Of New Eu Clinical Trials Regulation Framework On Translation Transperfect

Eu Clinical Trial Regulation Get Ready Set Go

European Clinical Trial Regulation No 536 2014 A5
Clinical Trial Regulation In Eu
What You Need To Know About The Eu Clinical Trial Regulation Pharmafile

Eu Explains How Info Requests Will Be Managed Under Clinical Trials Regulation Pink Sheet

Clinical Trial Disclosure Summary Of The Main Requirements In Eu Eea Download Table
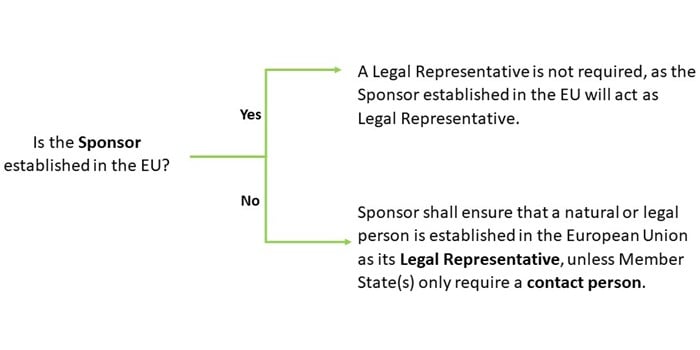
Legal Representation Set Out In The Eu Clinical Trial Regulation
